Chemistry
Chemistry
Explore Chapters
Combine Harvester, Cyclones, Damage caused by a cyclone, Hand Picking Technique, Magnetic Separation, Mixture, Properties of Mixture, Pure Substances, Requirement of Separation of Mixtures, Safety Measures During a Storm, Sieving Technique, Storms, Threshing, Tornadoes, Types of Mixtures, Winnowing Technique
In this section of Matter in Our Surroundings Classification of matter, Compressibility, Density, Evaporation causes cooling, Expansion or contraction, Factors affecting evaporation, Fluidity, General Properties of Liquids, General Properties of Solids, Matter is made up of particles, Melting point and boiling point, Particles of matter are too small to be imagined, Particles of matter attract each other, Particles of matter have spaces between them, Particles of some matter are in constant motion, Physical nature of Matter, Shape and Volume, etc. have been explained. Watch the videos and master the concepts
In this section of Matter in Our Surroundings Absorption of Heat, Boiling and boiling point, Bose Einstein Condensate, Condensation of gases, Difference between Gas and Vapour, Difference between steam and boiling water, Diffusion of gases, Effect of heating and cooling, Freezing, General Properties of Gases, Interconvertibility of the States of Matter, Measurement of temperature, Plasma state of matter, Pressure exerted by gas, Vaporization, etc. have been explained. Watch the videos and master the concepts
A pure substance consists of a single type of particles. Most of the matter around us exist as mixtures of two or more pure components, for example, sea water, minerals, soil etc. are all mixtures. In this section of Is Matter Around Us Pure Aqueous and Non Aqueous Solution, Colloids, Compound, Concentration of Solution Percentage by Mass, Concentration of Solution Percentage by Volume, Effect of Temperature and Pressure on Solubility, Elements, Mixture, Non Aqueous Solution, Physical and Chemical Change, Properties of Mixture, Pure Substances, Saturated and Unsaturated Solution, Saturated Solution, Solubility, Solute and Solvent, Solutions, Solvents, Suspension, True Solutions, Tyndal Effect, etc. have been explained. Watch the videos and master the concepts
In this Section of Atom, Molecule and ions we will read Atomic Mass Unit, Atomicity, Atoms, Characteristics of Atoms, Compound Molecules, Elementary Molecules, Formula of Binary Compounds, Gram Atomic Mass, Ions, Law of Conservation of Mass or Matter, Law of Constant or Definite Proportion, Laws of Chemical Combination, Molecule, Postulates of Dalton Atomic Theory, Properties of Molecules, Relative Atomic Mass, Rules for assigning Symbols, Size of an Atom, Types of Molecules, Valency, Valency of an Ion, Variable Valency, etc., Watch the videos and master the concepts
Explore Concepts (Click & View)
- Pure Substances

- Mixture

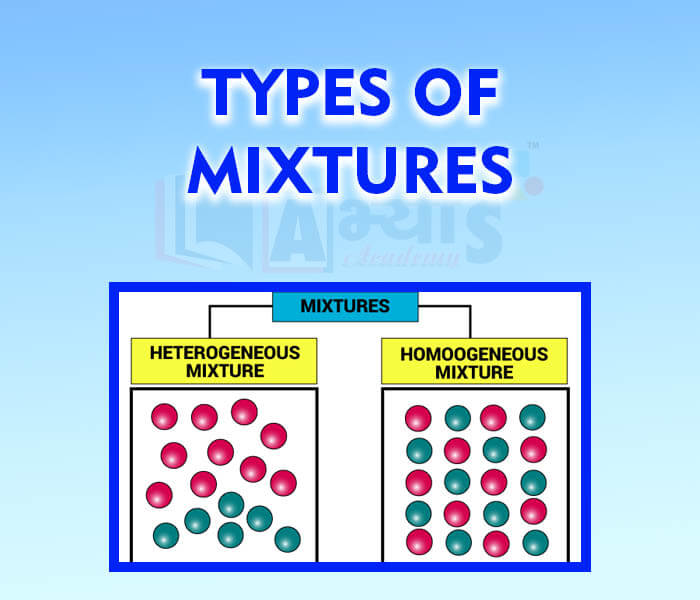
- Types of Mixtures
- Properties of Mixture

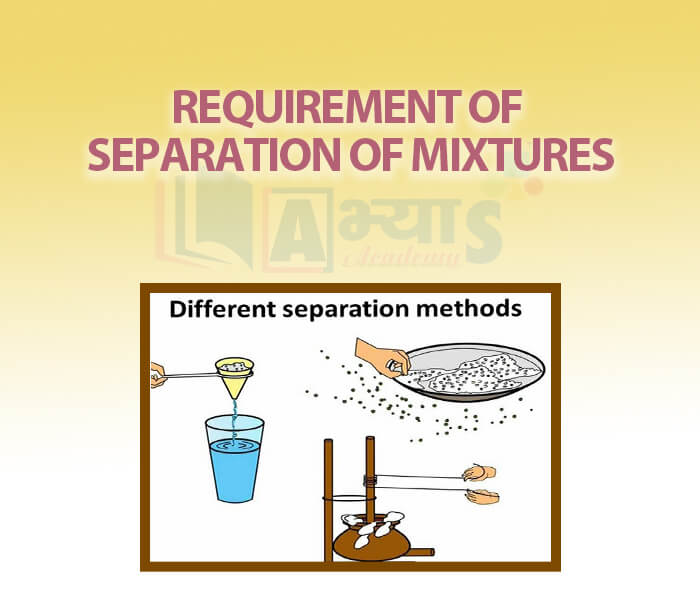
- Requirement of Separation of Mixtures
- Hand Picking Technique

- Threshing

- Winnowing Technique

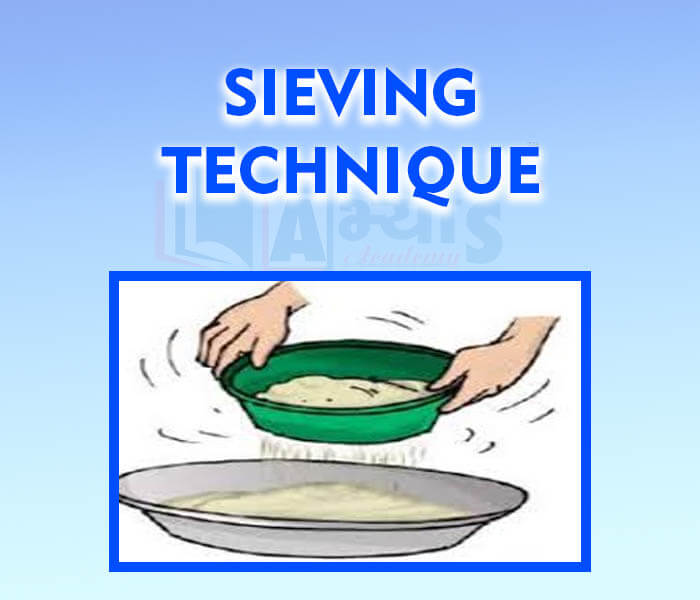
- Sieving Technique
- Combine Harvester

- Magnetic Separation

Explore Concepts (Click & View)
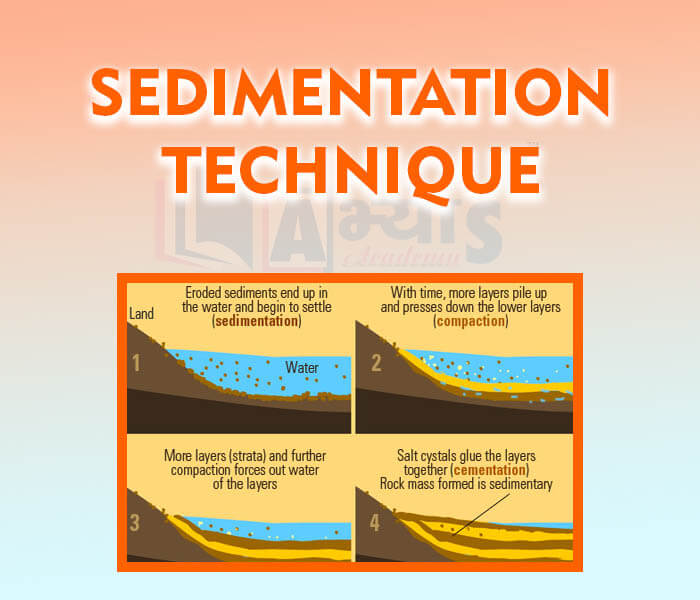
- Sedimentation Technique
- Decantation Technique
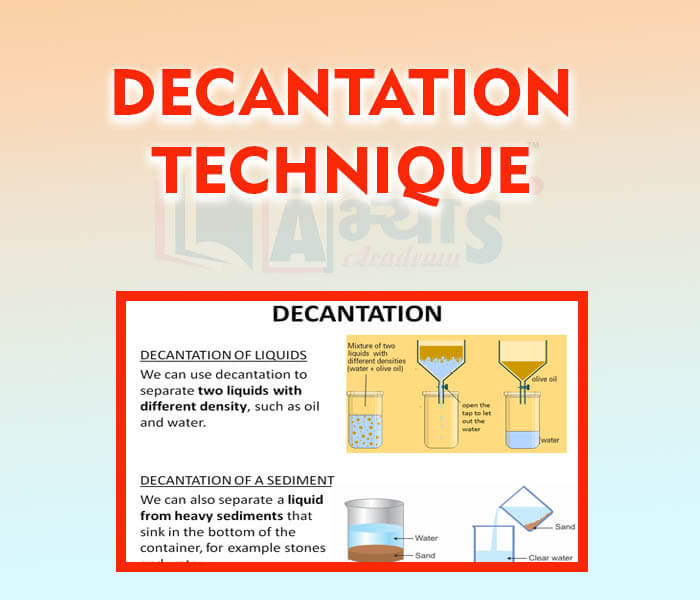
- Filtration Technique

- Purification of water

- Evaporation Technique

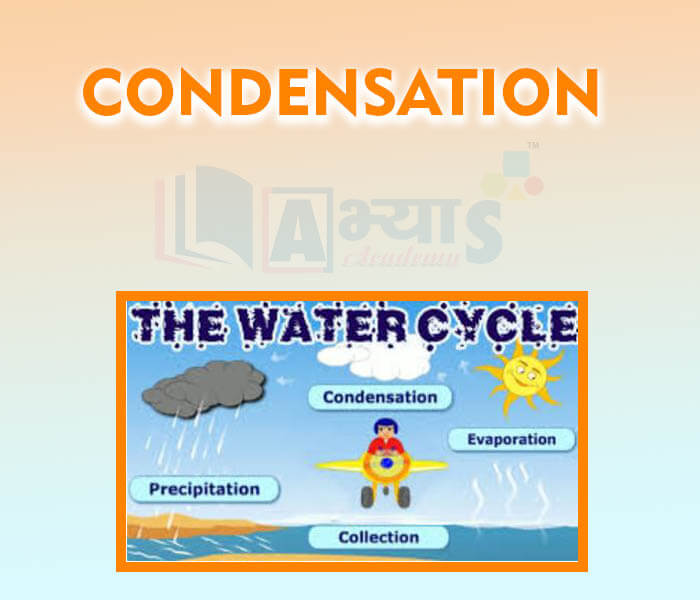
- Condensation
- Distillation Technique

- Solutions

- Solute and Solvent

- Saturated and Unsaturated Solution

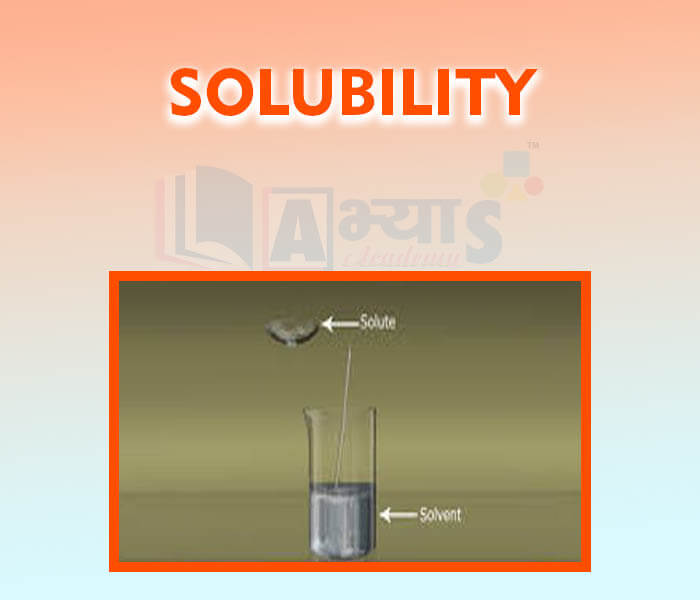
- Solubility
Explore Concepts (Click & View)
- Substance
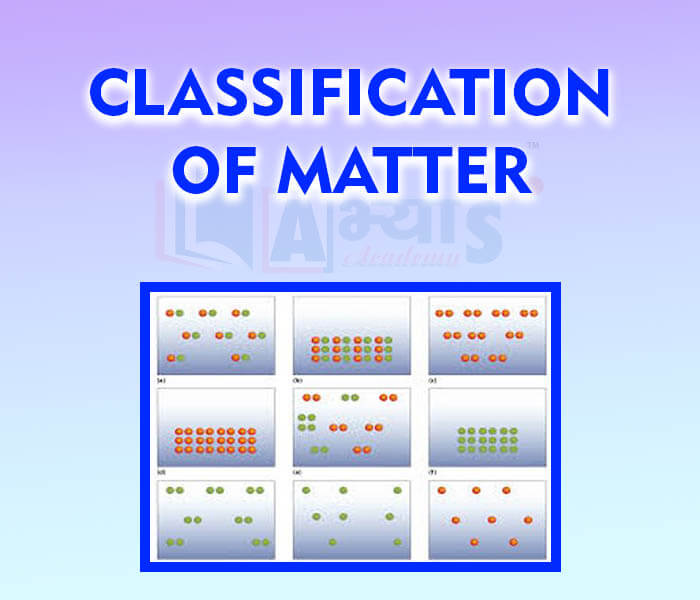
- Classification of matter
- Physical nature of Matter

- Matter is made up of particles

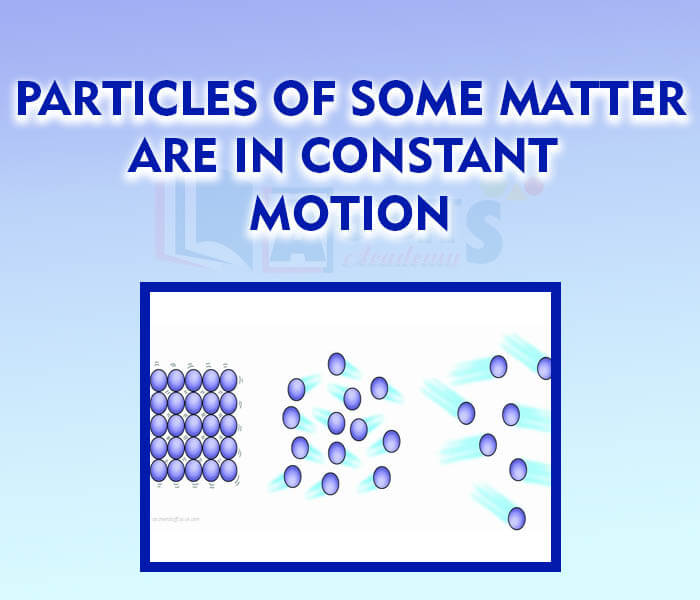
- Particles of some matter are in constant motion
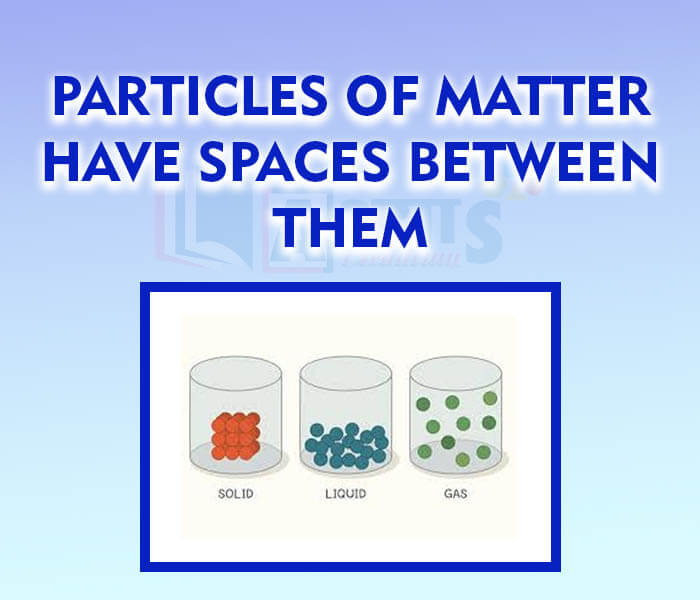
- Particles of matter have spaces between them
- General Properties of Solids

- General Properties of Liquids

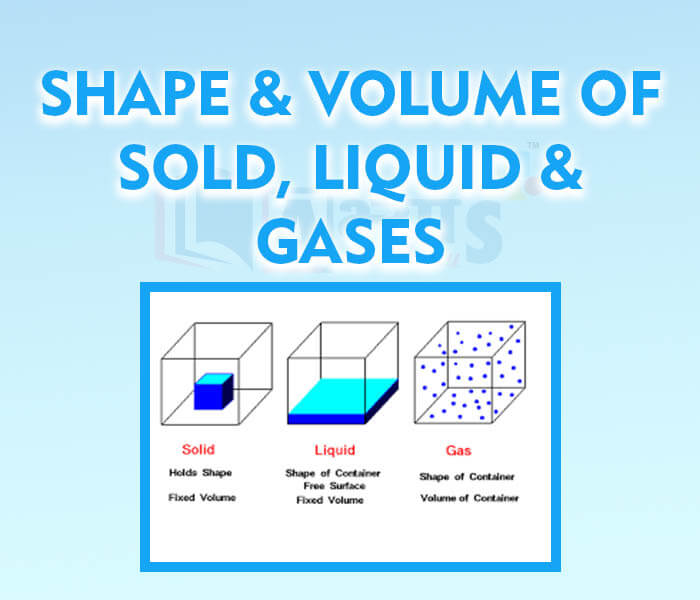
- Shape and Volume of Solid Liquid and Gases
- General Properties of Gases
Explore Concepts (Click & View)
- Physical and Chemical Change
- Elements

- Compound
Explore Concepts (Click & View)
- Symbols of Atoms and elements
- Rules for assigning Symbols
Students / Parents Reviews [10]
It was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thMy experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thAbout Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.

Shreya Shrivastava
8thBeing a parent, I saw my daughter improvement in her studies by seeing a good result in all day to day compititive exam TMO, NSO, IEO etc and as well as studies. I have got a fruitful result from my daughter.

Prisha Gupta
8thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.




