Mass Number






Mass Number
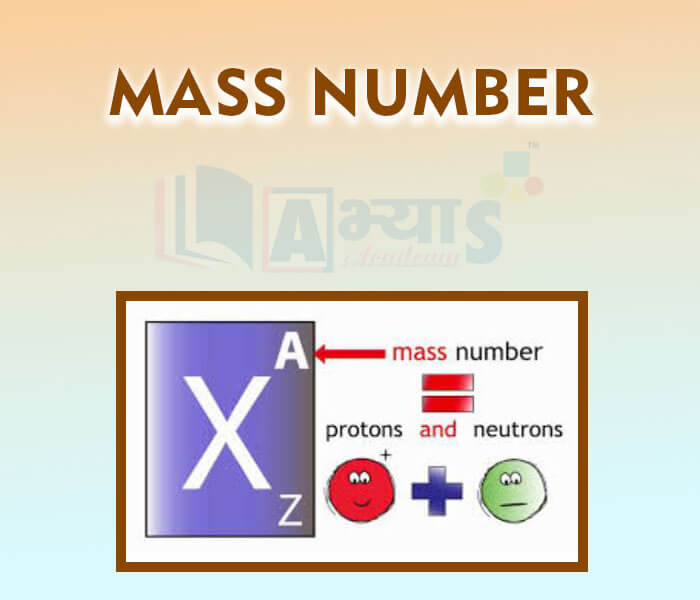
Mass Number: It is defined as the sum of number of protons and neutrons in the nucleus of an atom. Protons and neutrons together are called as nucleons. Mass number is denoted by A. Mass number (A) = Number of protons + number of neutrons. Eg: He, Li, At. mass = 4 and 7 for He and Li respectively.
Number of neutrons = Mass number - atomic number
Atomic number = Number of protonsMass number is written as a superscript towards the left of the symbol. In the notation for an atom, the atomic number, mass and symbol of the element are to be written as:
Here, the mass number of helium, lithium, sodium are 1, 7, 23 respectively.
If atomic number and mass number of an element are 11 and 23 respectively, What will be the number of neutrons present in it? | |||
| Right Option : B | |||
| View Explanation | |||
Identify the number of neutrons present in pair of elements with atomic numbers 20 and 13, if their mass numbers are 40 and 27, respectively. | |||
| Right Option : D | |||
| View Explanation | |||
Identify the number of neutrons present in a pair of elements with atomic numbers 20 and 13, if their mass numbers are 40 and 27, respectively: | |||
| Right Option : D | |||
| View Explanation | |||
Students / Parents Reviews [10]
One of the best institutes to develope a child interest in studies.Provides SST and English knowledge also unlike other institutes. Teachers are co operative and friendly online tests andPPT develope practical knowledge also.

Aman Kumar Shrivastava
10thIt was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thMy experience was very good with Abhyas academy. I am studying here from 6th class and I am satisfied by its results in my life. I improved a lot here ahead of school syllabus.

Ayan Ghosh
8thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thBeing a parent, I saw my daughter improvement in her studies by seeing a good result in all day to day compititive exam TMO, NSO, IEO etc and as well as studies. I have got a fruitful result from my daughter.
