| e-NOTES (1616 [C] ) | ||||||||||
| 8th (Chemistry) | |||||||||||
| Coal and Petroleum | |||||||||||
FuelsFuels: Substances that produce heat and light energy on burning are called fuels e.g. wood, coal kerosene LPG and petrol. Fuel is a source of energy and to obtain this heat energy, we have to burn the fuel. This burning is called oxidation. On the basis of physical state, fuels can be classified as solid liquid and gaseous. Liquid and gaseous fuels are preferred for domestic purposes like kerosene oil. Care should be taken so that complete combustion takes place. Incomplete combustion results in wastage of fuel and atmospheric pollution.
| |||||||||||
Characteristics of FuelsCharacteristics of Fuels: 1. Calorific value: When one kilogram of fuel is completely burnt in the presence of pure oxygen is called calorific value of fuel. It is expressed in unit’s kilojoule per gram (kJ/g). Higher the CV of a fuel the more heat it produces when burned. 2. Efficiency: When a fuel is burnt, some of the energy produced is given off as waste heat which cannot be used for cooking or other purposes. This is the effects of a fuel. It is expressed as percentage. 3. Ignition Temperature: The minimum temperature at which a fuel catches fire is termed as its ignition temperature. Those substances having low ignition temperature catches fire easily. Some important characteristics of an ideal/good fuel: No fuel is considered as ideal. Yet CNG is the best fuel among all others. Some fuels based on these characteristics: 1. Hydrogen as fuel: it is the best fuel and has highest calorific value. It is only used as a fuel because it is highly inflammable. 2. Methane and LPG as fuels: Both have high calorific values they burn with a smokeless fire and do not cause pollution. 3. Petrol and diesel as fuels: Both are used in auto mobiles their main disadvantage is their limited availability and other is that their combustion releases harmful gases. | |||||||||||
Fossil FuelsFossil fuels: Fossil fuels are formed from the buried remains of plants and animals over a period of millions of years. They produce large amount of energy on burning. Natural resources are divided into two types: Advantages of Fossil Fuel: Limitation of Fossil Fuels: Inexhaustible renewable resourcs : Those natural resourcs which can be replenished or reproduced easily. For example, sunlight is a resource which will never run out as the sun is expected to last for another 5 billion years. Oxygen is renewable because it is replaced in the atmosphere as plants release oxygen during photosynthesis. Many of the inexhaustible resources gets replenished with time quickly. But some of the inexhaustible resources are also depleting as they take longer time to replenish like ground water etc. Exhaustible resources : Those natural resources that get replenished for mankind. For example sources like fossil fuels, top fertile layer of soil, minerals, forests etc. Coal, Petreloum and natural gas: They are very important natural resources, and play a vital role in modern society. They are found in the earth's crust Their easy availability and specific characteristics make them very important in the growth of industry. At present they are the chief sources of energy worldwide. | |||||||||||
CoalCoal: Coal is black or brownish black in color. It is one of the oldest fuels. It is mainly made up of carbon along with other elements mainly hydrogen, sulphur, oxygen. Carbonisation : The process of coal formation is known as carbonisation. The dead plants and vegetation due to temperature and high pressure over hundreds of years slowly turned into coal. This slow conversion of dead plants and forests into coal is called the process of carbonisation. Formation of coal: Trees and other plants grown in swampy areas around 300 to 400 years ago died and remains got buried. In low oxygen conditions, high temperatures and absence of air, they formed a dark brown material called peat. Peat was compressed between layers of sediments and formed lignite. Even further compression resulted in formation of Anthracite. Depending on its carbon content it can be divided into three main types: 1. Anthracite: It has carbon content 92-98% and mainly used for heating. It is hard, black and glossy and is the source of any other fuel. 2. Bituminous coal: It has carbon content 60-80% and mainly used in heat and power generation and manufacturing of coke. 3. Lignite: It has carbon content around 25-35% and has lowest grade of coal. Commonly called brown coal. It is highly volatile and used in power generations. Coal is processed further to obtain useful materials by destructive distillation(heating of coal at high temperature in absence of air in controlled environment). | |||||||||||
Coal and PetroleumCoal : Coal is a mineral of dark brown or black colour formed from the remains of plants buried in the earth's crust millions years ago. It is very pure form of carbon. It mainly consists of carbon, hydrogen and oxygen. The coal deposits are spread over in the state of Jharkhand, Madhya Pardesh and West Bengal. Destructive distillation of coal: The process of heating coal in the absence of air is called the destructive distillation of coal. Coal contains a number of elements such as carbon, hydrogen, oxygen, nitrogen and sulphur. When coal is heated in the absence of air, a number of products are obtained. Peat has least carbon content and Anthracite has most carbon content. The main products obtained by the destructive distillation of coal are as follows: Coke: It is a hard, solid residue left behind destructive distillation of coal. It is pure form of carbon and used in steel manufacture, in power generations. Coal fields found near Bokaro and Jharia in Jharkhand. Coal tar: Thick, black, opaque liquid obtained as by product of the process of destructive distillation of coal. manufacturing coke. Used in antidandruff shampoos, ointments, soaps, perfumes, and making naphthalene balls. Coal gas: Gaseous fuel obtained as a by product of the process of manufacture coke. It is mainly composed of methane. Coal gas, gaseous mixture - mainly hydrogen, methane, and carbon monoxide - formed by the destructive distillation of coal and used as a fuel. .Ammonical Liquor : The ammonia produced as a result of destructive distillation of coal is absorbed in water. The aqueous solution of ammonia, i.e. ammonium hydroxide solution is called ammonical liquor. It is used in the preparation of fertilizers such as ammonium sulphate and ammonium superphosphate. Uses : Petroleum : Petroleum is a naturally occuring oil that consists of hydrocarbons with some other elements, such as sulphur, oxygen and nitrogen. It is also called as rock oil. Refining of petroleum : Petroleum is mixture of several hydrocarbons. It also contains water, salt and rocky materials. It cannot be used as crude.The process of seperating different components of petroleum is called refining of petroleum. This is done by the process of fractional distillation. The components obtained after fractional distillation are asphalt, lubricating oil, paraffin wax, fuel oil, diesel, kerosene, petrol and petroleum gas. Pollution by Coal & Petroleum: When coal and petroleum are burnt, the products like carbon dioxide, water, oxides of nitrogen and oxides of sulphur are released into air. When combustion takes place in insufficient air (oxygen), carbon monoxide is formed instead of carbon dioxide. The oxides of sulphur, nitrogen and carbon monoxide are poisonous at high concentrations among these products - Carbon dioxide is a greenhouse gas. The increase in amount of carbon dioxide in the atmosphere will lead to intense global warming. Thus, we need to use these resources judiciously. Conservation of Coat and Petroleum: General practices to reduce the consumption of coal petroleum are as follows: • Switch off the lights, fans, television and electrical appliances when not needed. • Use energy efficient appliances to save electricity. • Use stairs to climb at least up to three floors a building. • Public transport system needs to be improved, so that people can use them instead of using their personal vehicles. • On cold days, an extra sweater can be used instead of heating device like heater or sigri.
| |||||||||||
Petroleum and Natural GasPetroleum and Natural Gas: Petroleum and natural gas were formed from the remains of tiny marine organism that died millions of years ago. Process of formation of petroleum and natural gas: Tiny marine organisms died and settled on the ocean floor. With passage of time, this layer of dead organisms were covered beneath sediments. Enormous heat and pressure transformed the remains to petroleum and natural gas. Rising through porous rocks like sandstone, petroleum and natural gas reached a layer of impermeable rock and trapped below it. Petroleum and natural gas are extracted by drilling through the impermeable rocks. Petroleum is a complex nature of solid, liquid and gaseous hydrocarbon. The process of separating various constituents of petroleum is known as refining and carried out in refinery. It is heated to 400°C and introduced in fractionating column. Vapours of petroleum rise inside the fractionating column cool and condense at different heights, depending on their boiling paints and collected in different trays. Products obtained are: Natural Gas: It is a naturally occurring hydrocarbon gas mixture consisting mainly of methane and some other hydrocarbons as well. Uses of natural gas: Refining of petroleum : Petroleum is a mixture of several hydrocarbons. It is a foul - smelling brown black liquid. It also contains water, salt and rocky materials. It can not be used in this crude form either as a fuel or as a basic material to produce other useful components. Before being put to use, it has to be purified and refined. The process of separating the various components of petrol from one another is known as the refining of petroleum. This is done by a process called fractional distillation which is based on the fact that the different components of petroleum have distinctly different boiling points. They are seprated in a large fractionating column. Crude oil is pipped to the refinery from a well. It is washed with acid and alkali solutions to remove the basic and acidic impurities respectively. Crude oil is now heated about 673 K and fed at the base of fractionating column. All the components of expect asphalt are in the vapour state. As the mixture of hot vapours rise up in the column, it begins to cool. The components with the highest boiling point condense later. The components obtained at different heights in order from the bottom are asphalt, lubricating oil, paraffin wax, fuel oil, diesel, kerosene, petrol and petroleum gas. Products obtained on fractional distillation of crud : | |||||||||||
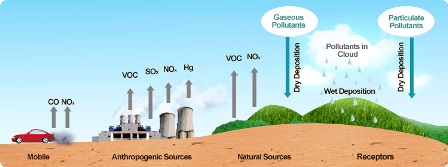
Pollution caused by burning of Fossil FuelsFossil fuels: generated from the decomposition of plant and animal matter over millions of years. Coal, oil, and natural gas are fossil fuels. Use of fossil fuels adds many undesirable substances called pollutants. These adversely affect the life of organisms. Carbon fuels such as wood, coal , petroleum release unburnt carbon particles in the environment. These particles are very dangerous pollutants and cause respiratory diseases for example asthma.Some of the main air pollutants are : Acidic Rain : When fossil fuels are burned, they release nitrogen oxides into the atmosphere, which contribute to the formation of acid rain. Oxides of sulphur and nitrogen dissolve in rain water and form acid. Such rain is called acidic rain. It causes skin and breathing problems. Global Warming : The phenomenon due to which excessive heat is trapped in the atmosphere because of excessive carbon dioxide, which lead to melting of ice caps, glaciers and resulting in weather changes. Higher rise in sea level : With increased global warming melting of glaciers is also occuring at high speed. This melting will result in the flooding of water bodies .The life at the coastal areas get affected the most. Oil spills : Materials like petroleum transported from one place to other with the help of tanks and ship. Any leakage in these tankers causes oil spills. This issue leads to the water pollution and it become difficult for aquatic organisms to survive. | |||||||||||
How to Conserve Coal and PetroleumCoal and petroleum are imporatnt non renewable fossil fuel. There increasing demand and usage is resulting in depletion of these useful resources. Conservation of these resources help us to preserve them for future generation. Conservation helps us to reduce the pollution, save money and provide longer life span to the resources. Conservation of fossil fuels can be done by: | |||||||||||
 | |||||||||||

Powered by ABHYAS ChatBOT.

What describes you best?

I am a Student
I am a Tutor
I am a School Owner
I want to appear for Govt. Exams